Supplements
Label claims for dietary supplements.
Dietary Supplements Labels Overview
Dietary supplements include any product taken by mouth that contains a “dietary ingredient,” which can be a vitamin, a mineral, an amino acid, or other herbs or botanicals. Dietary supplement labels look different from other food labels. Rather than an ordinary nutrition facts panel, dietary supplements must have a “Supplement Facts” panel.
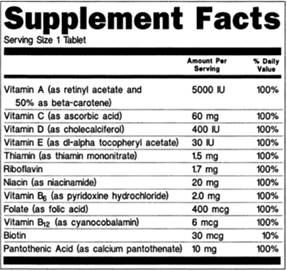
Sample “Supplement Facts” panel from FDA website:
Sample “Nutrition Facts” panel from FDA website:
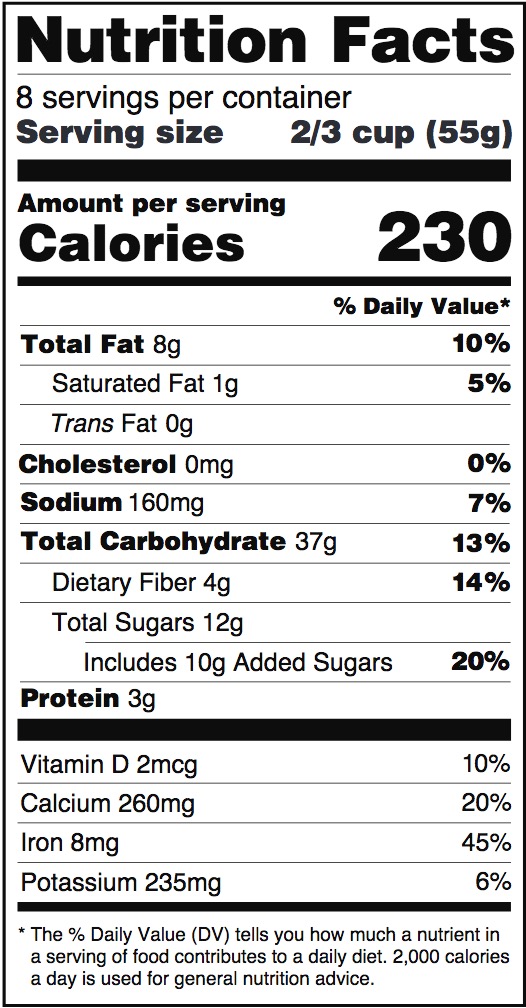
There are several notable differences between “Supplement Facts” and “Nutrition Facts” panels. Because dietary supplements are products that include dietary ingredients, a Supplement Facts panel is focused on listing those, which include vitamins, minerals, amino acids, and herbs or other botanicals. Nutrition Facts panels cannot list dietary ingredients beyond those specifically approved for the Nutrition Facts panel. Second, a Supplement Facts panel can list the source of the ingredient (e.g., “as folic acid”), as well as the part of a plant an ingredient is derived from, but a Nutrition Facts panel cannot. Finally, Nutrition Facts panels must list zero amounts of nutrients, but Supplement Facts panels cannot list zero amounts. For more information on these distinctions, see FDA Dietary Supplement Labeling guidance (note the guidance is current as of 2018, but includes some information that may not be current).
Generally, Supplement Facts panels must include the names and quantities of dietary ingredients, serving size, and servings per container. However, if the quantity of dietary ingredients is the same information as the servings per container, then the “Servings Per Container” information may be excluded.
Additionally, like the Nutrition Facts panel, Supplement Facts panels must include the following nutrients if they are present in measurable amounts: total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrates, dietary fibers, total sugars, added sugars, protein, Vitamin D, calcium, iron, and potassium.
Popular dietary ingredients and their synonyms: Vitamin C (ascorbic acid), Vitamin B1 (thiamin), Vitamin B2 (riboflavin), folate (folacin or folic acid), calories (energy) (energy might also be expressed in kilojoules).
Interactive Label
100% Natural
FDA does not have a formal definition for what this claim means, but it has acknowledged a “longstanding policy” concerning the use of the word “natural” in food labeling. FDA considers it to mean that nothing artificial or synthetic (including color additives) has been included in or added to a food if it would not normally be expected to be found in that food. FDA also states the use of the word “natural” currently does not address food production or processing methods, such as the use of pesticides, thermal technologies, pasteurization, or irradiation. FDA has not considered whether “natural” describes nutritional benefits.No Added Sugar
This label indicates that a manufacturer did not add any sugars to the product during processing. It does not mean that there is no sugar in the product, and the product could still contain naturally occurring sugars. See FDA regulations for more information.Soy-Free
The term “soy free” is not specifically regulated. However, under the Food Allergen Labeling and Consumer Protection Act of 2004, producers must include on the label whether the product contains a major food allergen, including soy.Dairy-Free
This term is not specifically regulated. But, like all label claims, the claim “dairy free” cannot be false or misleading. Additionally, milk ingredients need to be disclosed under the Food Allergen Labeling and Consumer Protection Act of 2004. The FDA does regulate the similar “non-dairy” claim.100% Vegan
FDA does not define this term, but it is generally used to mean foods made without any animal products like milk, honey, whey, or eggs. Vegan Action is a third-party organization that certifies products that do not contain animal products or byproducts and have not been tested on animals.Supports Muscle Recovery
A label that indicates what a product is supposed to do is referred to as a structure/function claim. A structure/function claim describes the role of a nutrient “intended to affect the normal structure or function of the human body.” FDA does not allow supplement manufacturers to make claims about a product’s ability to actually treat or prevent disease, so companies tend to stick to these more general claims about health. The manufacturer must submit proof that the claim is truthful and not misleading to FDA within 30 days of marketing the supplement. Additionally the label must include a disclaimer that FDA has not evaluated the claim. “The disclaimer must also state that the dietary supplement product is not intended to ‘diagnose, treat, cure or prevent any disease,’ because only a drug can legally make such a claim.”
Dietary Supplements Label Claims
FDA does not have a formal definition for what this claim means, but it has acknowledged a “longstanding policy” concerning the use of the word “natural” in food labeling. FDA considers it to mean that nothing artificial or synthetic (including color additives) has been included in or added to a food if it would not normally be expected to be found in that food. FDA also states the use of the word “natural” currently does not address food production or processing methods, such as the use of pesticides, thermal technologies, pasteurization, or irradiation. FDA has not considered whether “natural” describes nutritional benefits.
Dietary supplement labels may include cautionary statements, but the absence of one does not mean that there are no adverse side effects associated with the product. More information is available in this FDA guidance document.
Dairy Free
This term is not specifically regulated. But, like all label claims, the claim “dairy free” cannot be false or misleading. Additionally, milk ingredients need to be disclosed under the Food Allergen Labeling and Consumer Protection Act of 2004. The FDA does regulate the similar “non-dairy” claim.
Non-Dairy
Non-dairy foods may be made from animal proteins and contain milk products. When “non-dairy” labeled foods contain a caseinate ingredient (milk protein), the caseinate ingredient must be followed by a statement identifying its source. “For example, if the manufacturer uses the term ‘non-dairy’ on a creamer that contains sodium caseinate, it shall include a parenthetical term such as ‘a milk derivative’ after the listing of sodium caseinate in the ingredient list.” See FDA regulations for more information.
A dietary ingredient is a vitamin, mineral, herb, botanical, amino acid, or any other dietary substance intended for use by humans to supplement the diet. It can also be any concentrate, metabolite, constituent, extract, or combination of any of the above dietary ingredients. For more information, the statutory definition see here. Additional information is also available in this FDA guidance document.
The FDA defines the term “dietary supplement” as “a product taken by mouth that contains a dietary ingredient intended to supplement the diet.” A dietary supplement often comes in the form of a pill, liquid, or powder. They can also be in other forms like a bar, if the product does not represent itself as a “conventional food or a sole item of a meal or diet.” For more information see FDA Regulations.
FDA warns to watch out for claims such as “quick and effective,” “cure all,” or “totally safe.” These labels are not regulated by FDA and before consuming products with these labels, one should consult with a health professional.
FDA does not “approve” dietary supplement facilities or manufacturers. FDA does regulate manufacturing practices and has the authority to regularly inspect facilities to ensure they are in compliance with the law. However, these inspections do not result in a certification of “FDA Approved,” and there is no official seal for products to indicate that they are produced in a facility that is in compliance with FDA regulations.
If an ingredient is present in any amount that cannot be declared as zero then it is present in a measurable amount. For example, if dietary fiber is present in an amount greater than 1 gram, it is present in a measurable amount. If there are fewer than 5 calories, then a label may read zero calories. For more information on measurable amounts see FDA regulations.
This label indicates that a manufacturer did not add any sugars to the product during processing. It does not mean that there is no sugar in the product, and the product could still contain naturally occurring sugars. See FDA regulations for more information.
This term includes dietary ingredients that have recommended daily intakes or daily recommended values; this term is listed in the Supplement Facts panel following the list of dietary ingredients with daily values. More information is available in the FDA Dietary Supplement Labeling Guide.
Over-the-counter supplements can never be “pharmaceutical strength.” A supplement of the same strength as a pharmaceutical substance would be considered a drug, and subject to additional regulations.
This label indicates that the manufacturer has mixed several ingredients into a blend used only by that manufacturer, and the manufacturer does not need to state exactly how much of each ingredient is included. However, the ingredients will still be listed in order of the amount that the product contains. Proprietary blends are discussed further in FDA regulations.
The serving size for a dietary supplement is the maximum amount recommended on the label for consumption per eating occasion. FDA designates the serving size and label statement units for specific foods as well as larger categories of foods. If there is no recommendation, then the serving size is one unit, which could mean anything from one tablet to a spoonful of the contents of the container. Whichever unit is decided must be included on the label. If the recommended serving size is a range (1-3 tablets, for example) then the serving size is the upper end of that range (3 tablets). For more information on serving sizes, see FDA regulations. More information is available in this FDA guidance document.
The term “soy free” is not specifically regulated. However, under the Food Allergen Labeling and Consumer Protection Act of 2004, producers must include on the label whether the product contains a major food allergen, including soy.
A label that indicates what a product is supposed to do is referred to as a structure/function claim. A structure/function claim describes the role of a nutrient “intended to affect the normal structure or function of the human body.” FDA does not allow supplement manufacturers to make claims about a product’s ability to actually treat or prevent disease, so companies tend to stick to these more general claims about health. The manufacturer must submit proof that the claim is truthful and not misleading to FDA within 30 days of marketing the supplement. Additionally the label must include a disclaimer that FDA has not evaluated the claim. “The disclaimer must also state that the dietary supplement product is not intended to ‘diagnose, treat, cure or prevent any disease,’ because only a drug can legally make such a claim.”
This term means that a product has been produced according to the standards in the Organic Foods Production Act (OFPA) and certified by USDA’s National Organic Program. A label may only use the “USDA Organic” seal if the food is actually certified organic.
Organic products are grown and processed according to federal standards on soil quality, pest and weed control, and use of chemical fertilizers, among other areas. According to USDA, “[o]rganic producers rely on natural substances and physical, mechanical, or biologically based farming methods to the fullest extent possible.”
The amount of organic ingredients in a food matters for labeling. A “100 percent organic” product may only have ingredients that are organic. A food that has at least 95 percent organic ingredients may have the term “organic” on its label if the organic ingredients are specifically noted somewhere on the package. For more information on organic ingredient labeling, see the USDA Organic Labeling Standards website.
FDA does not define this term, but it is generally used to mean foods made without any animal products like milk, honey, whey, or eggs. Vegan Action is a third-party organization that certifies products that do not contain animal products or byproducts and have not been tested on animals.
Q&As
- Does FDA approve dietary supplements before they are on the market?
- What are the ingredients in my supplement other than the specific dietary ingredients/vitamins that I am taking it for?
- How are dietary ingredients ordered in their listing on the Supplement Facts panel?
- Why do dietary supplement labels look different from other food labels?
-
Does FDA approve dietary supplements before they are on the market?
FDA generally is not authorized to review dietary supplements before they are marketed, because dietary supplements are not drugs. To remove a dietary supplement from the market, the FDA has to show that the product is “adulterated” (typically meaning the product contains a substance that is harmful to health) or “misbranded” (the labeling is false or misleading).
Back to questions
-
What are the ingredients in my supplement other than the specific dietary ingredients/vitamins that I am taking it for?
Binders and fillers are added to supplements to stabilize them and make them a solid, easily ingestible product. Coloring agents may also be added to dietary supplements. All substances added must be preapproved as safe for use by FDA or generally recognized as safe (GRAS) before being included in a dietary supplement.
Back to questions
-
How are dietary ingredients ordered in their listing on the Supplement Facts panel?
Ingredients should be listed in descending order of predominance by weight, and vitamins, minerals, and electrolytes are grouped together. For information, see FDA’s Dietary Supplement Labeling Guide.
Back to questions
-
Why do dietary supplement labels look different from other food labels?
There are several major differences between Supplement Facts and Nutrition Facts. These differences include:
- Dietary ingredients without recommended daily intake values or daily reference values established by FDA must be included on the Supplement Facts panel of dietary supplements, but are not permitted to be included on the Nutrition Facts panel for foods. (21 CFR 101.36(b)(2)-(3))
- The source of a dietary ingredient may be listed on the Supplement Facts panel of a dietary supplement, but may not be listed on the Nutrition Facts panel for a food. If the source of a dietary ingredient is listed on the Supplement Facts panel, then the source does not need to be included in the ingredient statement for dietary supplements. (21 CFR 101.4(h))
- The part of a plant from which a dietary ingredient was derived must be included in the Supplement Facts panel of a dietary supplement, but the part of the plant may not be included in the Nutrition Facts panel for foods. (21 CFR 101.36(d)-(d)(1))
- If there are amounts of nutrients in a dietary supplement that would be listed as zero, that information should not be included on the Supplement Facts panel. This information must be included on the Nutrition Facts panel for foods. (21 CFR 101.9)
For more information about dietary supplement labels, see FDA’s Dietary Supplement Labeling Guide.
Back to questions
Additional Resources on Dietary Supplements Labeling
FDA Dietary Supplement Labeling
This FDA page provides regulatory information for dietary supplement labeling, catered to dietary supplement producers. The question/answer format offers information ranging from how dietary supplements are defined, label statement details, and other miscellaneous FDA requirements and regulations for dietary supplement producers.
FDA Nutrition Labeling
This FDA page provides regulatory information for nutrition labeling, catered to producers. The question/answer format offers information categorized by serving size, nutrient declaration, amounts, percent of daily value, dietary ingredients, format, compliance, exemptions, and special labeling provisions.
FDA 101: Dietary Supplements
For more information on dietary supplements generally, visit this FDA website with helpful guidance, links, and explanations.
Dietary Supplement Labeling Guide
Visit this FDA guide for more information on the differences between labeling dietary supplements and food nutrition labels.
Nutrition Facts Labeling Requirements
This document provides additional examples of Supplement Facts labels.
